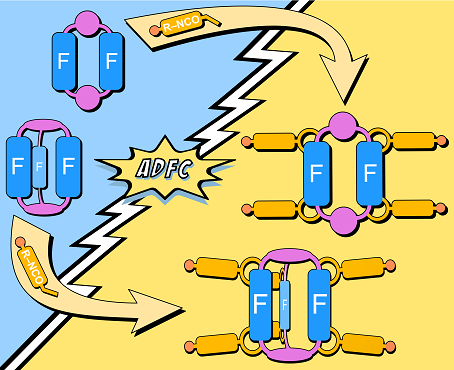
A project several years in the making using our FPOCs finally saw the light of day! We present the multiple post-modification of organic macrocycles and cages, introducing functional groups into two- and three-dimensional supramolecular scaffolds bearing fluorine substituents, which opens up new possibilities in multi-step supramolecular chemistry employing the vast chemical space of readily available isocyanates. The mechanism and scope of the reaction that proceeds after isocyanate addition to the benzylamine motif via an azadefluorination cyclisation (ADFC) were investigated using DFT calculations, and a series of aromatic isocyanates with different electronic properties were tested. The compounds show excellent chemical stability and were fully characterised. They can be used for subsequent cross-coupling reactions, and ADFC can be used directly to generate cross-linked membranes from macrocycles or cages when using ditopic isocyanates.
Congratulations, especially to Tobi, Tim, and Tom, for putting in the hard work! Click here to read the original article online.